Opioid-Induced Autonomic Disruption
Our research investigates how opioids, particularly fentanyl, disrupt autonomic regulation by altering vagal nerve activity. Opioid overdose is a leading cause of preventable death, primarily due to impaired breathing and cardiovascular control. We focus on the vagus nerve — a critical pathway for autonomic communication between the brain and vital organs — to uncover how opioids compromise respiratory rhythms and airway function. Using advanced in vivo neural recordings, respiratory measurements, and targeted interventions, we are identifying the neural mechanisms by which opioids destabilize the balance of autonomic control. Our work aims to develop novel strategies to protect vital functions during opioid exposure, providing a foundation for safer therapeutic approaches and emergency interventions.
Opioids and Obstructions
Opioid overdose is a leading cause of preventable death, primarily due to impaired breathing and cardiovascular control. This project focuses on the vagus nerve — a critical pathway for autonomic communication between the brain and vital organs — to uncover how opioids compromise respiratory rhythms and airway function. Using advanced in vivo neural recordings, respiratory measurements, and targeted interventions, we are identifying the neural mechanisms by which opioids destabilize the balance of autonomic control. Our work aims to develop novel strategies to protect vital functions during opioid exposure, providing a foundation for safer therapeutic approaches and emergency interventions.
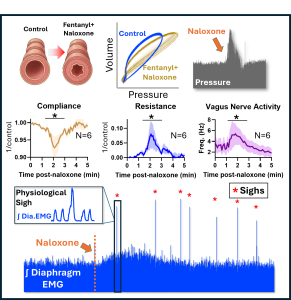
Ketamine as an alternative to Fentanyl
Disrupted Brainstem Signaling
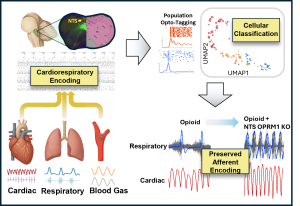
Opioids profoundly disrupt breathing, yet the network-level mechanisms within the nucleus tractus solitarius (NTS), the brainstem hub for integrating cardiorespiratory afferents, remain poorly understood. To remedy this disparity we use high-density Plexon SiNAPS recordings measure neuronal activity in the NTS simultaneously with physiological variables including respiratory airflow, diaphragm EMG, end-tidal CO2 and pulse pressure. We utilize modeling to parse out clusters of similar neurons and quantify how individual neurons encode respiratory phase, respiratory drive, blood pressure, CO2, and cardiac phase. Our findings thus-far support a model in which opioid-induced dysregulation of NTS afferent integration contributes to failures of compensatory homeostatic reflexes during opioid overdose.